Structure and Bonding | Organic Chemistry 1
Atomic Structure
X: Symbol of the element
Z: Atomic number = number of protons
A: Mass number = number of protons + number of neutrons
Number of neutrons = A-Z
Number of electrons = number of protons - charge
Z is always the same for a specific element
A can be different. and are two isotopes
Electron Configuration
Electron configuration:
The arrangement of electrons in the atomic orbitals of an atom. An atomic orbital can hold a maximum of 2 electrons
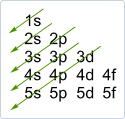
The electronic configuration of an element can easily be determined by using the mnemonic below. The diagonal lines give the order of the subshells. The maximum number of electrons in each subshell is as follows: 2 e- in the s-subshells, 6 e- in the p-subshells, 10 e- in the d-subshells, 14 e- in the f-subshells
Oxygen ⇒ Z = 8 and neutral atom ⇒ 8 electrons ⇒ 1s22s22p4
Iron ⇒ Z = 26 and neutral atom ⇒ 26 electrons ⇒ 1s22s22p63s23p64s23d6
Ionic and Covalent Bonds
The octet rule:
The general rule governing the bonding process for second-row elements (the main elements in organic chemistry): atoms tend to form molecules in such a way that they reach an octet in the valence shell and reach a noble gas configuration
Ionic bonds vs. covalent bonds
Bonding is a process of joining 2 atoms that results in a decrease in energy and an increase in stability: the atoms get a complete outer shell of valence electrons. There are 2 main types of bonds:
- Ionic bonds are based on the strong electrostatic attraction between 2 ions of opposite charges. These bonds result from the transfer of electrons from one element to another in order to follow the octet rule
- Covalent bonds are bonds with 2 electrons. These bonds result from the sharing of electrons between 2 atoms (especially those in the middle of the periodic table). The electrons are shared to allow the atoms to reach noble-gas configurations
Expected number of covalent bonds around an atom = 8 - number of valence electrons
Carbon: Z = 6 ⇒ 6 e- and neutral ⇒ 1s2 2s2 2p2 ⇒ 4 e- in its valence shell
Carbon needs 4 more e- to get the configuration of Neon and will therefore form 4 covalent bonds with other atoms ⇒ carbon atom is tetravalentNitrogen: Z = 7⇒ 7 e- and neutral ⇒ 1s2 2s2 2p3 ⇒ 5 e- in its valence shell
Nitrogen needs 3 more e- to get the configuration of Neon and will therefore form 3 covalent bonds with other atoms ⇒ nitrogen is trivalent
Formal Charge
Formal charge:
Charge assigned to individual atoms in a Lewis structure
Formal charge = number of valence electrons in free atom - number of valence electrons in bound atom
Number of valence electrons in bound atom = number of unshared electrons + number of shared electrons
What are the formal charges in the CH3NO2 molecule?
N: 1s2 2s2 2p3 = [He] 2s2 2p3 ⇒ 5 valence electrons in free atom
4 bonds: 8 shared electrons ⇒ 4 valence electrons in bound atom
Formal charge = 5 - 4 = + 1O: 1s2 2s2 2p4 = [He] 2s2 2p4 ⇒ 6 valence electrons in free atom
2 bonds + 2 lone pairs: 4 shared e- + 4 unshared e- ⇒ 6 valence e- in bound atom
Formal charge = 6 - 6 = 0O: 1s2 2s2 2p4 = [He] 2s2 2p4 ⇒ 6 valence electrons in free atom
1 bond + 3 lone pairs: 2 shared e- + 6 unshared e- ⇒ 7 valence e- in bound atom
Formal charge = 6 - 7 = - 1
Hybridization and Geometry
Hybridization:
The combination of 2 or more atomic orbitals to form the same number of hybrid orbitals:
- sp3 hybridization: the combination of 1 s-orbital and 3 p-orbitals to form 4 sp3 hybrid orbitals
- sp2 hybridization: the combination of 1 s-orbital and 2 p-orbitals to form 3 sp2 hybrid orbitals
- sp hybridization: the combination of 1 s-orbital and 1 p-orbital to form 2 sp hybrid orbitals
Geometry:
The number of electron domains (atoms or lone pairs of electrons) around an atom determines its geometry and hybridization:
- 2 electron domains ⇒ linear (bond angle: 180°) ⇒ sp hybridization
- 3 electron domains ⇒ trigonal planar (bond angle: 120°) ⇒ sp2 hybridization
- 4 electron domains ⇒ tetrahedral (bond angle: 109.5°) ⇒ sp3 hybridization
Resonance Forms
Resonance structures:
A group of Lewis structures with the same placement of the atoms but a different placement of their π or nonbonded electrons ⇒ their single bonds remain the same but the position of their multiple bonds and nonbonded electrons differ. Resonance structures must be valid Lewis structures
The different resonance forms of a substance are not all equal: the form with the most bonds and fewer charges has a higher contribution to the resonance hybrid
Selecting the best resonance structure that contributes the most to the resonance hybrid:
- Lower formal charges (positive or negative) are preferable to higher charges
- Formal charges on adjacent atoms are not desirable
- A more negative formal charge should reside on a more electronegative atom
Difference between isomers and resonance structures:
2 isomers differ by the arrangement of their atoms and electrons, while 2 resonance structures differ only by the arrangement of their electrons
Lewis Structures
Lewis structure:
A representation of the arrangement of atoms and the position of all valence electrons in a molecule or polyatomic ion. Shared electron pairs are represented by lines between 2 atoms, and lone pairs are represented by pairs of dots on individual atoms. We always try to satisfy the octet rule (or duet rule for hydrogen) when writing Lewis structures
- Dot: one nonbonding electron
- Pair of dots: lone electron pair (lone pair)
- Line: two shared electrons (bond)
Lewis structure of NH3:N: 5 valence electrons ⇒ needs 3 shared electrons ⇒ 3 covalent bonds
H: 1 valence electron ⇒ needs 1 shared electron ⇒ 1 covalent bond
Lewis structure of CO2:C: 4 valence electrons ⇒ needs 4 shared electrons ⇒ 4 covalent bonds
O: 6 valence electrons ⇒ needs 2 shared electrons ⇒ 2 covalent bonds
How to write Lewis structures:
- Count the total number of valence electrons. Add or subtract electrons if you have a negative or a positive charge
- Determine the number of covalent bonds / lone pairs the molecule will have by dividing the number of valence electrons by 2. Use the molecular formula to draw the skeletal structure
- Distribute the remaining valence electrons to satisfy the octet rule, completing the octet of the more electronegative atoms first. Include double or triple bonds if necessary
- Check the number of valence electrons in the drawn molecule
- Assign formal charges to all atoms
Draw a Lewis structure for methanol CH3OH
Count the valence electrons:
1 C x 4 e- + 4 H x 1 e- + 1 O x 6 e- = 14 valence electrons ⇒ 7 bonds and lone pairsArrange the atoms
Add bonds ...
5 bonds ⇒ 10 electrons
... then lone pairs
5 bonds + 2 lone pairs ⇒ 14 e-
Shorthand Representations
Shorthand methods are used to abbreviate the structure of organic molecules. The 2 main types of shorthand representations are:
Condensed structure:
- The main carbon chain is written horizontally. The atoms are drawn next to the atoms to which they are bonded
- Covalent bonds and lone pairs are omitted
- Parentheses are used around similar groups bonded to the same atom. If different substituents are bonded to the same atom, vertical lines can be used
Skeletal structure:
- Carbon atoms are not shown: it is assumed that a carbon is at the junction of 2 lines and at the end of any line
- The hydrogens around each carbon are not drawn, but we assume that there are enough hydrogens for the carbons to follow the octet rule
- All the heteroatoms are drawn as well as the hydrogens that are directly bonded to them
Check your knowledge about this Chapter
X: Symbol of the element
Z: Atomic number = number of protons
A: Mass number = number of protons + number of neutrons
The number of neutrons in an atom is equal to the mass number (A) of the atom minus the number of protons (Z)
The number of electrons in an atom is equal to the number of protons (Z) minus the charge of the atom
No, the number of protons determines the element and is therefore specific for an element and determines the element. The mass number (A) of an element can be different. This is the case between 2 isotopes
An atomic orbital can hold a maximum of 2 electrons. Thus:
- s subshell: 1 s orbital ⇒ 2 electrons
- p subshell: 3 p orbitals ⇒ 6 electrons
- d subshell: 5 d orbitals ⇒ 10 electrons
- f subshell: 7 f orbitals ⇒ 14 electrons
The order of orbital energies is: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s ... You can easily remember this order by using the mnemonic on the right:
The octet rule states that atoms tend to form molecules in such a way that they reach an octet in the valence shell and reach a noble gas configuration. The octet rule applies to almost all compounds made up of second period elements
Bonding is a process of joining 2 atoms that results in a decrease in energy and an increase in stability: the atoms get a complete outer shell of valence electrons. There are 2 main types of bonds: ionic bonds and covalent bonds
- Ionic bonds are based on the strong electrostatic attraction between 2 ions of opposite charges. These bonds result from the transfer of electrons from one element to another in order to follow the octet rule
- Covalent bonds are bonds with 2 electrons. These bonds result from the sharing of electrons between two atoms (especially those in the middle of the periodic table). The electrons are shared to allow the atoms to reach noble-gas configurations
The expected number of covalent bonds around an atom is equal to 8 minus the number of valence electrons of the atom
The formal charge of an atom is the number of valence electrons in the free atom minus the number of valence electrons in the bound atom. The number of valence electrons in the bound atom is equal to the number of unshared electrons + number of shared electrons
Hybridization is the combination of 2 or more atomic orbitals to form the same number of hybrid orbitals:
- sp3 hybridization: the combination of 1 s-orbital and 3 p-orbitals to form 4 sp3 hybrid orbitals
- sp2 hybridization: the combination of 1 s-orbital and 2 p-orbitals to form 3 sp2 hybrid orbitals
- sp hybridization: the combination of 1 s-orbital and 1 p-orbital to form 2 sp hybrid orbitals
The number of electron domains (atoms or lone pairs of electrons) around an atom determines its geometry and hybridization:
- 2 electron domains ⇒ linear (bond angle: 180°) ⇒ sp hybridization
- 3 electron domains ⇒ trigonal planar (bond angle: 120°) ⇒ sp2 hybridization
- 4 electron domains ⇒ tetrahedral (bond angle: 109.5°) ⇒ sp3 hybridization
Resonance structures are a group of Lewis structures with the same placement of the atoms but a different placement of their π or nonbonded electrons ⇒ their single bonds remain the same but the position of their multiple bonds and nonbonded electrons differ
The different resonance forms of a substance are not all equal: the form with the most bonds and less charges has a larger contribution to the resonance hybrid
Principles for determining which resonance structure is most stable:
- Lower formal charges (positive or negative) are preferable to higher charges
- Formal charges on adjacent atoms are not desirable
- A more negative formal charge should reside on a more electronegative atom
2 isomers differ by the arrangement of their atoms and electrons, while 2 resonance structures differ only by the arrangement of their electrons
A Lewis structure is a representation of the arrangement of atoms and the position of all valence electrons in a molecule or polyatomic ion. Shared electron pairs are represented by lines between 2 atoms, and lone pairs are represented by pairs of dots on individual atoms
- Count the total number of valence electrons. Add or subtract electrons if you have a negative or positive charge
- Determine the number of covalent bonds / lone pairs the molecule will have by dividing the number of valence electrons by 2. Use the molecular formula to draw the skeletal structure
- Distribute the remaining valence electrons to satisfy the octet rule, completing the octet of the more electronegative atoms first. Include double or triple bonds if necessary
- Check the number of valence electrons in the drawn molecule
- Assign formal charges to all atoms
- The main carbon chain is written horizontally. The atoms are drawn next to the atoms to which they are bonded
- Covalent bonds and lone pairs are omitted
- Parentheses are used around similar groups bonded to the same atom. If different substituents are bonded to the same atom, vertical lines can be used
- Carbon atoms are not shown: it is assumed that a carbon is at the junction of 2 lines and at the end of any line
- The hydrogens around each carbon are not drawn, but we assume that there are enough hydrogens for the carbons to follow the octet rule
- All the heteroatoms are drawn as well as the hydrogens that are directly bonded to them








