Quiz 3 - Chemical Kinetics: Mechanisms | Chemical Kinetics: Mechanisms
General Chemistry 2 - Quiz 3 - Chemical Kinetics: Mechanisms
In which ways may a catalyst increase the rate of a reaction?
I. It may alter the rate law of the reaction.
II. It may make the overall reaction more exothermic.
A catalyst can alter the rate law of a reaction by introducing a new reaction pathway with a different mechanism. This new mechanism can have a different rate-determining step, which may change the rate law.
However, a catalyst does not make the overall reaction more exothermic or endothermic; it does not change the enthalpy of the reaction. The catalyst only affects the reaction rate by lowering the activation energy.
The rate constant of a chemical reaction increases by 63% when the temperature is raised from 40 oC to 45 oC. What is the activation energy of this reaction?
To solve this, we can use the Arrhenius equation in its logarithmic form:
ln k = - + ln A
k = rate constant
A = frequency factor (also known as the pre-exponential factor)
Ea = activation energy (in J.mol-1)
R = molar gas constant (8.314 J.mol-1.K-1)
T = temperature (in K)
Therefore,
ln =
k1 and k2 are the rate constants at temperatures T1 and T2
R is the gas constant (8.314 J.mol-1.K-1)
T11 and T2 are the initial and final temperatures in Kelvin
Given:
- The rate constant increases by 63%, so = 1.63
- T1 = 40 + 273.15 = 313.15 K
- T2 = 45 + 273.15 = 318.15 K
ln (1.63) = ⇒ 0.489 = Ea x 6.036 x 10-6
Ea = 80.9 x 103 J.mol-1 = 80.9 kJ.mol-1
The activation energy of a certain reaction is 87 kJ.mol-1. What is the ratio of the rate constants for this reaction when the temperature is decreased from 37 oC to 15 oC?
To solve this, we can use the Arrhenius equation in its logarithmic form:
ln k = - + ln A
k = rate constant
A = frequency factor (also known as the pre-exponential factor)
Ea = activation energy (in J.mol-1)
R = molar gas constant (8.314 J.mol-1.K-1)
T = temperature (in K)
Therefore,
ln =
k1 and k2 are the rate constants at temperatures T1 and T2
Ea is the activation energy (87 kJ.mol-1 = 87,000 J.mol-1)
R is the gas constant (8.314 J.mol-1.K-1)
T1 and T2 are the initial and final temperatures in Kelvin
Given:
- T1 = 37 + 273.15 = 310.15 K
- T2 = 15 + 273.15 = 288.15 K
ln = = -2.576
= e-2.576 =
The reaction: CHCl3 (g) + Cl2 (g) → CCl4 (g) + HCl (g) is believed to proceed by the following mechanism:
Cl2 (g) → 2 Cl (g) (fast)
Cl (g) + CHCl3 (g) → HCl (g) + CCl3 (g) (slow)
CCl3 (g) + Cl (g) → CCl4 (g) (fast)
What rate equation is consistent with this mechanism?
The rate-determining step (slow step) in the mechanism is: Cl (g) + CHCl3 (g) → HCl (g) + CCl3 (g)
The rate law for the reaction is determined by the slowest step. According to the mechanism, the rate of the reaction should be proportional to the concentration of Cl and CHCl3: Rate = k [Cl][CHCl3]
However, Cl is not a reactant but an intermediate, and its concentration must be expressed in terms of the initial reactants. From the fast equilibrium step: Cl2 (g) → 2 Cl (g), the concentration of Cl can be related to [Cl2] as: [Cl] ∝ [Cl2].
Thus, the rate law becomes: Rate = k [Cl2]1/2[CHCl3]
Consider this reaction: 2 H2 (g) + 2 NO (g) → N2 (g) + 2 H2O (g). The rate law for this reaction is: Rate = k [H2][NO]2. Under what conditions could these steps represent the mechanism?
2 NO ⇌ N2O2
N2O2 + H2 → N2O + H2O
N2O + H2 → N2 + H2O
For the given rate law, Rate = k [H2][NO]2, the mechanism must produce a rate law consistent with this expression.
-
Step 1: 2 NO ⇌ N2O2 is a fast equilibrium step, so the concentration of N2O2 can be expressed in terms of [NO]2.
-
Step 2: If this step is slow, it will be the rate-determining step. The rate of this step would depend on the concentrations of N2O2 and H2, leading to the rate law Rate = k [N2O2][H2]. Since [N2O2] is proportional to [NO]2, the rate law becomes Rate = k [H2][NO]2, which matches the given rate law.
Therefore, the mechanism could be consistent with the observed rate law if step 2 is the slow, rate-determining step.
Which straight line gives the activation energy for a reaction?
According to the Arrhenius equation:
ln k = - + ln A
k = rate constant
A = frequency factor (also known as the pre-exponential factor)
Ea = activation energy (in J.mol-1)
R = molar gas constant (8.314 J.mol-1.K-1)
T = temperature (in K)
By measuring the rate constant k at different temperatures and plotting lnk versus T-1 (a plot called an Arrhenius plot), a straight line is obtained with a slope of - and an intercept of lnA.
When a catalyst is added to the system represented by this energy-reaction coordinate diagram, which dimensions in the diagram are changed?
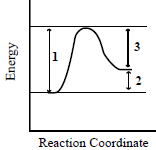
In the energy-reaction coordinate diagram:
- Dimension 1 represents the activation energy (the energy barrier that must be overcome for the reaction to proceed).
- Dimension 2 represents the difference in energy between the reactants and products (which is the enthalpy change, ΔH).
- Dimension 3 likely represents the energy difference between the activated complex (transition state) and the products.
When a catalyst is added:
- Dimension 1 (activation energy) decreases because the catalyst provides an alternative pathway with a lower activation energy.
- Dimension 2 (enthalpy change, ΔH) does not change because the catalyst does not affect the energy difference between the reactants and products.
- Dimension 3 (the difference between the transition state and products) is related to the activation energy, so it may also change depending on how the reaction pathway is altered by the catalyst.
The activation energy for a reaction can be determined by measuring the reaction rate at different:
According to the Arrhenius equation:
ln k = - + ln A
k = rate constant
A = frequency factor (also known as the pre-exponential factor)
Ea = activation energy (in J.mol-1)
R = molar gas constant (8.314 J.mol-1.K-1)
T = temperature (in K)
By measuring the rate constant k at different temperatures and plotting lnk versus T-1 (a plot called an Arrhenius plot), a straight line is obtained with a slope of - .