Does Shape Matter in Molecular Geometry?
The shape of a molecule helps determine its properties. For example, carbon dioxide is not soluble in water because its linear molecules don't fit into the polar environment well enough to get mixed up with other compounds.
What is the Difference Between Molecular Geometry and Molecular Shape?
The structure of a molecule, excluding lone pairs on central atoms. Geometry describes how electron arrangements around that particular atom are made up of other bonds or single groups in its composition. The basic molecular geometries include:
- Bent, Angular, or Non-Linear
- Linear
- Octahedral
- Pentagonal Bipyramidal
- See-Saw or Distorted Tetrahedron
- Square Planar
- Square Pyramidal
- T-shaped
- Tetrahedral
- Trigonal Planar
- Trigonal Pyramidal
- Trigonal Bipyramidal
What Are Bonding Patterns?
You will notice that chemists commonly use generic formulas to represent bonding patterns. These generic formulas are known as the "AXE Method."
- "A" represents the central atom
- "X" represents the bonding pairs
- "E" represents the lone pair of electrons
How Does Molecular Shape Affect Physical Properties?
The shape of a molecule has an effect on its polarity, which can be either positive or negative. Polar compounds dissolve in polar solutions and have higher boiling points than non-polar ones do; they also melt at reduced temperatures when compared with their unsaturated counterparts (which exist as solids). To find the particular shape of a molecule, you must first find the steric number.
What is the Steric Number in Chemistry?
The steric number (often abbreviated as SN, but not to be confused with "SN" which stands for "nucleophilic substitution") is the sum of the number of bonded atoms plus the number of lone pairs. VSEPR (valence shell electron pair repulsion) theory uses the steric number to determine the molecular geometry.
How Do I Find the Steric Number?
To find the steric number, you must first use the Lewis Structure. For example, the AX5 molecular shape has a steric number of 5 and is trigonal bipyramidal. This means it has 5 atoms bonded to the central atom. Here's a quick reference table to help you compare the differences between the steric numbers and how they affect molecular shape:
Steric Numbers
| Steric Number (SN) | Ideal Bond Angle | Shape | Lone Pairs (E) | Bonding Electron Pairs (X) | Polar/Non-Polar | Example |
| 2 (AX2) | 180o | Linear | 0 | 2 | Polar | CO2 |
| 3 (AX3) | 120o | Trigonal Planar | 0 | 3 | Non-Polar | BF3 |
| 4 (AX4) | 109.5o | Tetrahedral | 0 | 4 | Non-Polar | SiH4 |
| 5 (AX5) | 90o | Trigonal Bipyramidal | 0 | 5 | Non-Polar | PCI5 |
| 6 (AX6) | 90o | Octahedral | 0 | 6 | Non-Polar | SF6 |
1 Lone Pair of Electrons
| Steric Number (SN) | Ideal Bond Angle | Shape | Lone Pairs (E) | Bonding Electron Pairs (X) | Polar/Non-Polar | Example |
| 3 (AX2E) | <120o | Bent | 1 | 2 | Polar | SO2 |
| 4 (AX3E) | 109.5o | Pyramidal | 1 | 3 | Polar | NH3 |
| 5 (AX4E) | 120o | See-Saw | 1 | 4 | Polar | TeCl4 |
| 6 (AX5E) | 90o | Square Pyramidal | 1 | 5 | Polar | IF5 |
2 Lone Pairs of Electrons
| Steric Number (SN) | Ideal Bond Angle | Shape | Lone Pairs (E) | Bonding Electron Pairs (X) | Polar/Non-Polar | Example |
| 3 (AXE2) | 180o | Trigonal Planar | 2 | 1 | - | - |
| 4 (AX2E2) | 109.5o | Bent | 2 | 2 | Polar | H2O |
| 5 (AX3E2) | 120o | T-shaped | 2 | 3 | Polar | CIF3 |
| 6 (AX4E2) | 90o | Square Planar | 2 | 4 | Non-Polar | XeF4 |
3 Lone Pairs of Electrons
| Steric Number (SN) | Ideal Bond Angle | Shape | Lone Pairs (E) | Bonding Electron Pairs (X) | Polar/NonPolar | Example |
| 4 (AXE3) | 180o | Linear | - | - | - | - |
| 5 (AX2E3) | 120o | Linear | 3 | 2 | Non-Polar | I3- |
| 6 (AX3E3) | 180o | T-shaped | 3 | 3 | - | - |
4 Lone Pairs of Electrons
| Steric Number (SN) | Ideal Bond Angle | Shape | Lone Pairs (E) | Bonding Electron Pairs (X) | Polar/NonPolar | Example |
| 5 (AXE4) | 180o | Linear | 4 | 1 | - | - |
| 6 (AX2E4) | - | - | 4 | 2 | - | - |
Identifying the Molecular Shape by Steric Number
Another method to look at molecular geometry is by assigning the shape of a molecule according to its steric number.
For example:
SN2 = linear
SN3 = trigonal planar
SN4 = tetrahedral
SN5 = trigonal bipyramidal
SN6 = octahedral
VSEPR Rules
By following a few simple rules for VSEPR, you can identify the molecular shape much faster:
- Identify the central atom by drawing the Lewis Structure.
- Count the number of valence electrons and add one electron for each bonding atom.
- Add or subtract electrons for the charge.
- Divide the total of these by 2 to find the total number of electron pairs.
- Use this number to predict the shape.
Can Molecules Have More Than One Central Atom?
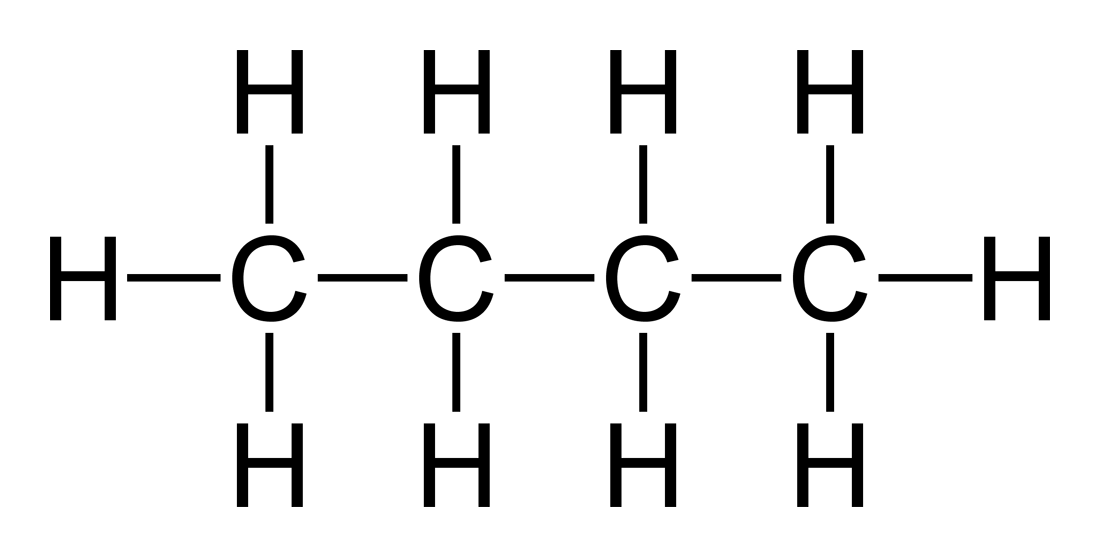
Yes. For example, butane has a molecular shape of C4H10. If we take this long-chain molecule and cut it up into different pieces, we see that each of the carbon atoms is its own central atom. Therefore, butane contains 4 central atoms.
Why Do Molecular Compounds Differ in Shape?
VESPR (valence shell electron pair repulsion) theory states that negatively charged electrons repel one another. The forces that make them repel one another distorts the bond angles, thus affecting the shape of molecules. The shapes of molecules in a compound are dictated by the VESPR theory. The shapes may be linear, trigonal pyramidal, tetrahedral, bent, or a combination of these. The electron pairs surrounding the central atom in a molecule take up different spatial arrangements in relation to each other that determine the shape of the compound. This means that the electron pairs do not interact with each other, but they interact with the central atom. This determines the shape of molecules in a compound.
How Do Multiple Bonds Affect the Shape of a Molecule?
Single bonds are stronger. Double and triple bonds repel one another stronger. Polarity in molecules determines their polarity and structure. The molecule's polar bonds and negative dipole moments determine polarity.
How Does Changing a Bond to a Double or Triple Bond Affect the Shape of the Molecules?
In VSEPR theory, the more bonds there are around an atom's central core electrons in a chemical bond, the more distortion in bond angles. In essence, multiple bonds simply take up more space, just like with lone pairs of electrons.